Energy density
Energy density is a term used for the amount of useful energy stored in a given system or region of space per unit volume.[1]
For fuels, the energy per unit volume is sometimes a useful parameter. In a few applications, comparing, for example, the effectiveness of hydrogen fuel to gasoline it turns out that hydrogen has a higher specific energy than does gasoline, but, even in liquid form, a much lower energy density.
Energy per unit volume has the same physical units as pressure, and in many circumstances is an exact synonym: for example, the energy density of the magnetic field may be expressed as (and behaves as) a physical pressure, and the energy required to compress a compressed gas a little more may be determined by multiplying the difference between the gas pressure and the pressure outside by the change in volume.
Contents |
Energy density in energy storage and in fuel
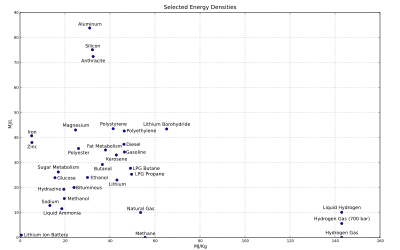
In energy storage applications the energy density relates the mass of an energy store to the volume of the storage equipment. The higher the energy density, the more energy may be stored or transported for the same amount of volume. In the context of fuel selection, that energy density of a fuel per unit mass is the specific energy of that fuel, though in general an engine using that fuel will yield less energy due to inefficiencies and thermodynamic considerations—hence the specific fuel consumption of an engine will be greater than the reciprocal of the specific energy of the fuel.
The highest density sources of energy are fusion and fission. Fusion includes energy from the sun which will be available for billions of years (in the form of sunlight) but humans have not learned to make our own sustained fusion power sources. Fission of U-235 in nuclear power plants will be available for billions of years because of the vast supply of the element on earth.[2] Coal, gas, and petroleum are the current primary energy sources in the U.S.[3] but have a much lower energy density. Burning local biomass fuels supplies household energy needs (cooking fires, oil lamps, etc.) worldwide.
Energy density (how much energy you can carry) does not tell you about energy conversion efficiency (net output per input) or embodied energy (what the energy output costs to provide, as harvesting, refining, distributing, and dealing with pollution all use energy). Like any process occurring on a large scale, intensive energy use impacts the world. For example, global warming, nuclear waste storage, and deforestation may be some of the consequences of supplying our growing energy demands from fossil fuels, nuclear fission, or biomass.
No single energy storage method boasts the best in specific power, specific energy, and energy density. Peukert's Law describes how the amount of useful energy that can be obtained (for a lead-acid cell) depends on how quickly we pull it out.
Gravimetric and volumetric energy density of some fuels and storage technologies (modified from the Gasoline article):
- Note: Some values may not be precise because of isomers or other irregularities. See Heating value for a comprehensive table of specific energies of important fuels.
True energy densities
This table gives the energy density of a complete system, including all required external components, such as oxidisers or heat sources. One MJ ≈ .28 KWh ≈ 0.37 HPh.
| Storage type | Specific energy (MJ/kg) | Energy density (MJ/L) | Peak recovery efficiency % | Practical recovery efficiency % |
|---|---|---|---|---|
| Indeterminate Antimatter | ≈89,876,000,000 | not applicable | ||
| Deuterium-tritium fusion | 576,000,000 | |||
| Uranium-235 used in nuclear weapons | 144,000,000 | 1,500,000,000 | ||
| Natural uranium (99.3% U-238, 0.7% U-235) in fast breeder reactor | 86,000,000[2] | |||
| Reactor-grade uranium (3.5% U-235) in light water reactor | 3,456,000 | 30% | ||
| Pu-238 α-decay | 2,200,000 | |||
| Hf-178m2 isomer | 1,326,000 | 17,649,060 | ||
| Natural uranium (0.7% U235) in light water reactor | 443,000 | 30% | ||
| Ta-180m isomer | 41,340 | 689,964 | ||
| Specific orbital energy of Low Earth orbit (approximate) | 33 | |||
| Beryllium + Oxygen | 23.9[4] | |||
| Lithium + Fluorine | 23.75 | |||
| Octaazacubane potential explosive | 22.9[5] | |||
| Dinitroacetylene explosive - computed | 9.8 | |||
| Octanitrocubane explosive | 8.5[6] | 16.9[7] | ||
| Tetranitrotetrahedrane explosive - computed | 8.3 | |||
| Heptanitrocubane explosive - computed | 8.2 | |||
| Sodium (reacted with chlorine) | 7.0349 | |||
| Hexanitrobenzene explosive | 7[8] | |||
| Tetranitrocubane explosive - computed | 6.95 | |||
| Ammonal (Al+NH4NO3 oxidizer) | 6.9 | 12.7 | ||
| Tetranitromethane + hydrazine bipropellant - computed | 6.6 | |||
| Nitroglycerin | 6.38[9] | 10.2[10] | ||
| ANFO-ANNM | 6.26 | |||
| Octogen (HMX) | 5.7[9] | 10.8[11] | ||
| TNT [Kinney, G.F.; K.J. Graham (1985). Explosive shocks in air. Springer-Verlag. ISBN 3-540-15147-8.] | 4.610 | 6.92 | ||
| Copper Thermite (Al + CuO as oxidizer) | 4.13 | 20.9 | ||
| Thermite (powder Al + Fe2O3 as oxidizer) | 4.00 | 18.4 | ||
| Hydrogen peroxide decomposition (as monopropellant) | 2.7 | 3.8 | ||
| battery, Lithium ion nanowire | 2.54 | 95%[12] | ||
| battery, Lithium Thionyl Chloride (LiSOCl2)[13] | 2.5 | |||
| Water 220.64 bar, 373.8°C | 1.968 | 0.708 | ||
| Kinetic energy penetrator | 1.9 | 30 | ||
| battery, Fluoride ion | 1.7 | 2.8 | ||
| battery, Hydrogen closed cycle H fuel cell[14] | 1.62 | |||
| Hydrazine(toxic) decomposition (as monopropellant) | 1.6 | 1.6 | ||
| Ammonium nitrate decomposition (as monopropellant) | 1.4 | 2.5 | ||
| Thermal Energy Capacity of Molten Salt | 1 | 98%[15] | ||
| Molecular spring approximate | 1 | |||
| battery, Sodium Sulfur | .72[16] | 1.23 | 85%[17] | |
| battery, Lithium-manganese[18][19] | 0.83-1.01 | 1.98-2.09 | ||
| battery, Lithium ion[20][21] | 0.46-0.72 | 0.83-3.6[22] | 95%[23] | |
| battery, Lithium Sulphur[24] | 1.80[25] | 1.80 | ||
| battery (Sodium Nickel Chloride), High Temperature | 0.56 | |||
| battery, Silver-oxide[18] | 0.47 | 1.8 | ||
| Flywheel | 0.36-0.5[26][27] | |||
| 5.56 × 45 mm NATO bullet | 0.4 | 3.2 | ||
| battery, Nickel metal hydride (NiMH), low power design as used in consumer batteries[28] | 0.4 | 1.55 | ||
| battery, Zinc-manganese (alkaline), long life design[18][20] | 0.4-0.59 | 1.15-1.43 | ||
| Liquid Nitrogen | 0.349 | |||
| Water - Enthalpy of Fusion | 0.334 | 0.334 | ||
| battery, Zinc Bromine flow (ZnBr)[29] | 0.27 | |||
| battery, Nickel metal hydride (NiMH), High Power design as used in cars[30] | 0.250 | 0.493 | ||
| battery, Nickel cadmium (NiCd)[20] | 0.14 | 1.08 | 80%[23] | |
| battery, Zinc-Carbon[20] | 0.13 | 0.331 | ||
| battery, Lead acid[20] | 0.14 | 0.36 | ||
| battery, Vanadium redox | 0.09 | 0.1188 | 70-75% | |
| battery, Vanadium Bromide redox | 0.18 | 0.252 | 80%–90%[31] | |
| Capacitor Ultracapacitor | 0.0199[32] | 0.050 | ||
| Capacitor Supercapacitor | 0.01 | 80%–98.5%[33] | 39%–70%[33] | |
| Superconducting magnetic energy storage | 0.008[34] | >95% | ||
| Capacitor | 0.002[35] | |||
| Neodymium magnet | 0.003[36] | |||
| Ferrite magnet | 0.0003[36] | |||
| Spring power (clock spring), torsion spring | 0.0003[37] | 0.0006 | ||
| Storage type | Energy density by mass (MJ/kg) | Energy density by volume (MJ/L) | Peak recovery efficiency % | Practical recovery efficiency % |
Energy densities ignoring external components
This table lists energy densities of systems that require external components, such as oxidisers or a heat sink or source. These figures do not take into account the mass and volume of the required components as they are assumed to be freely available and present in the atmosphere. Such systems cannot be compared with self-contained systems.
| Storage type | Specific energy (MJ/kg) | Energy density (MJ/L) | Peak recovery efficiency % | Practical recovery efficiency % | |
|---|---|---|---|---|---|
| Hydrogen, liquid | 143 | 10.1 | |||
| Hydrogen, compressed at 700 bar[38] | 143 | 5.6 | |||
| Hydrogen, gas | 143 | 0.01079 | |||
| Beryllium | 67.6 | 125.1 | |||
| Lithium borohydride | 65.2 | 43.4 | |||
| Boron[39] | 58.9 | 137.8 | |||
| Methane (1.013bar, 15°C) | 55.6 | 0.0378 | |||
| Natural gas | 53.6[40] | 0.0364 | |||
| LNG (NG at -160°C) | 53.6[40] | 22.2 | |||
| CNG (NG compressed to 250 bar ~3600 psi) | 53.6[40] | 9 | |||
| LPG propane [41] | 49.6 | 25.3 | |||
| LPG butane [41] | 49.1 | 27.7 | |||
| Gasoline (Petrol)[41] | 46.4 | 34.2 | |||
| Diesel fuel/residential heating oil [41] | 46.2 | 37.3 | |||
| Nitromethane | 11.3 | ||||
| Polyethylene plastic | 46.3[42] | 42.6 | |||
| Polypropylene plastic | 46.4[42] | 41.7 | |||
| 100LL Avgas | 44.0[43] | 31.59 | |||
| Gasohol E10 (10% ethanol 90% gasoline by volume) | 43.54 | 33.18 | |||
| Lithium | 43.1 | 23.0 | |||
| Jet A aviation fuel[44] / kerosene | 42.8 | 33 | |||
| Biodiesel oil (vegetable oil) | 42.20 | 33 | |||
| DMF (2,5-dimethylfuran) | 42[45] | 37.8 | |||
| Crude oil (according to the definition of ton of oil equivalent) | 46.3 | 37[40] | |||
| Polystyrene plastic | 41.4[42] | 43.5 | |||
| Body fat metabolism | 38 | 35 | 22[46] | ||
| Butanol | 36.6 | 29.2 | |||
| Gasohol E85 (85% ethanol 10% gasoline by volume) | 33.1 | 25.65 | |||
| Graphite | 32.7 | 72.9 | |||
| coal, Anthracite[47] | 32.5 | 72.4 | 36 | ||
| Silicon [48] | 32.2 | 75.1 | |||
| Aluminum | 31.0 | 83.8 | |||
| Ethanol | 30 | 24 | |||
| Polyester plastic | 26.0 [42] | 35.6 | |||
| Magnesium | 24.7 | 43.0 | |||
| coal, Bituminous[49] | 24 | 20 | |||
| PET plastic | 23.5 (impure)[50] | ||||
| Methanol | 19.7 | 15.6 | |||
| Hydrazine (toxic) combusted to N2+H2O | 19.5 | 19.3 | |||
| Liquid ammonia (combusted to N2+H2O) | 18.6 | 11.5 | |||
| PVC plastic (improper combustion toxic) | 18.0[42] | 25.2 | |||
| Peat briquette [51] | 17.7 | ||||
| Sugars, carbohydrates & protein metabolism | 17 | 26.2(dextrose) | 22[52] | ||
| coal, Lignite | 14.0 | ||||
| Calcium | 15.9 | 24.6 | |||
| Glucose | 15.55 | 23.9 | |||
| Dry cowdung and cameldung | 15.5[53] | ||||
| Wood [54] | 18.0 | ||||
| Sodium (burned to wet sodium hydroxide) | 13.3 | 12.8 | |||
| Household waste | 8.0[55] | ||||
| Sod peat | 12.8 | ||||
| Sodium (burned to dry sodium oxide) | 9.1 | 8.8 | |||
| Zinc | 5.3 | 38.0 | |||
| Teflon plastic (combustion toxic, but flame retardant) | 5.1 | 11.2 | |||
| iron (burned to iron(III) oxide) | 5.2 | 40.68 | |||
| iron (burned to iron(II) oxide) | 4.9 | 38.2 | |||
| battery, Lithium-Air rechargeable | 3.6[56] | ||||
| battery, Zinc air[57] | 1.59 | 6.02 | |||
| Liquid nitrogen | 0.77[58] | 0.62 | |||
| Compressed air at 300 bar (potential energy) | 0.5 | 0.2 | 50+% | ||
| Latent heat of fusion of Ice (Thermal) | 0.335 | 0.335 | |||
| Water at 100 m dam height (potential energy) | 0.001 | 0.001 | 85-90% | ||
| Storage type | Energy density by mass (MJ/kg) | Energy density by volume (MJ/L) | Peak recovery efficiency % | Practical recovery efficiency % |
Energy density of electric and magnetic fields
Electric and magnetic fields store energy. In a vacuum, the (volumetric) energy density (in SI units) is given by
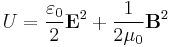 ,
,
where E is the electric field and B is the magnetic field. In the context of magnetohydrodynamics, the physics of conductive fluids, the magnetic energy density behaves like an additional pressure that adds to the gas pressure of a plasma.
In normal (linear) substances, the energy density (in SI units) is
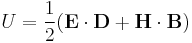 ,
,
where D is the electric displacement field and H is the magnetizing field.
Energy density of empty space
In physics, "vacuum energy" or "zero-point energy" is the volumetric energy density of empty space. More recent developments have expounded on the concept of energy in empty space.
Modern physics is commonly classified into two fundamental theories: quantum field theory and general relativity. Quantum field theory takes quantum mechanics and special relativity into account, and it's a theory of all the forces and particles except gravity. General relativity is a theory of gravity, but it is incompatible with quantum mechanics. Currently these two theories have not yet been reconciled into one unified description, though research into "quantum gravity" and, more recently, stochastic electrodynamics, seeks to bridge this divide.
In general relativity, the cosmological constant is proportional to the energy density of empty space, and can be measured by the curvature of space.
Quantum field theory considers the vacuum ground state not to be completely empty, but to consist of a seething mass of virtual particles and fields. These fields are quantified as probabilities—that is, the likelihood of manifestation based on conditions. Since these fields do not have a permanent existence, they are called vacuum fluctuations. In the Casimir effect, two metal plates can cause a change in the vacuum energy density between them which generates a measurable force.
Some believe that vacuum energy might be the "dark energy" (also called Quintessence) associated with the cosmological constant in general relativity, thought to be similar to a negative force of gravity (or antigravity). Observations that the expanding universe appears to be accelerating seem to support the cosmic inflation theory—first proposed by Alan Guth in 1981—in which the nascent universe passed through a phase of exponential expansion driven by a negative vacuum energy density (positive vacuum pressure).
See also
- Power density and specifically
- Power-to-weight ratio
- Figure of merit
- Energy content of biofuel
- Heat of combustion
- Heating value
- Rechargeable battery
- Specific impulse
- Vacuum energy
External references
Zero point energy
- Eric Weisstein's world of physics: energy density[59]
- Baez physics: Is there a nonzero cosmological constant?[60]
- Introductory review of cosmic inflation[61]
- An exposition to inflationary cosmology[62]
Density data
- ^ "Aircraft Fuels." Energy, Technology and the Environment Ed. Attilio Bisio. Vol. 1. New York: John Wiley and Sons, Inc., 1995. 257–259
- “Fuels of the Future for Cars and Trucks” - Dr. James J. Eberhardt - Energy Efficiency and Renewable Energy, U.S. Department of Energy - 2002 Diesel Engine Emissions Reduction (DEER) Workshop San Diego, California - August 25–29, 2002
Energy storage
Books
- The Inflationary Universe: The Quest for a New Theory of Cosmic Origins by Alan H. Guth (1998) ISBN 0-201-32840-2
- Cosmological Inflation and Large-Scale Structure by Andrew R. Liddle, David H. Lyth (2000) ISBN 0-521-57598-2
- Richard Becker, "Electromagnetic Fields and Interactions", Dover Publications Inc., 1964
Footnotes
- ↑ http://physics.nist.gov/Pubs/SP811/sec04.html
- ↑ 2.0 2.1 "Facts from Cohen". Formal.stanford.edu. 2007-01-26. http://www-formal.stanford.edu/jmc/progress/cohen.html. Retrieved 2010-05-07.
- ↑ "U.S. Energy Information Administration (EIA) - Annual Energy Review". Eia.doe.gov. 2009-06-26. http://www.eia.doe.gov/emeu/aer/pecss_diagram.html. Retrieved 2010-05-07.
- ↑ "The Heat of Formation of Beryllium Oxide1 - Journal of the American Chemical Society (ACS Publications)". Pubs.acs.org. 2002-05-01. http://pubs.acs.org/doi/abs/10.1021/ja01109a018. Retrieved 2010-05-07.
- ↑ "Besides N2, What Is the Most Stable Molecule Composed Only of Nitrogen Atoms?† - Inorganic Chemistry (ACS Publications)". Pubs.acs.org. 1996-05-28. http://pubs.acs.org/doi/abs/10.1021/ic9606237. Retrieved 2010-05-07.
- ↑ http://www3.interscience.wiley.com/journal/122324589/abstract
- ↑ "Octanitrocubane - Wikipedia, the free encyclopedia". En.wikipedia.org. http://en.wikipedia.org/wiki/Octanitrocubane. Retrieved 2010-05-07.
- ↑ http://www3.interscience.wiley.com/journal/109618256/abstract
- ↑ 9.0 9.1 "Chemical Explosives". Fas.org. 2008-05-30. http://www.fas.org/man/dod-101/navy/docs/es310/chemstry/chemstry.htm. Retrieved 2010-05-07.
- ↑ Česky. "Nitroglycerin - Wikipedia, the free encyclopedia". En.wikipedia.org. http://en.wikipedia.org/wiki/Nitroglycerin. Retrieved 2010-05-07.
- ↑ Česky (2010-05-01). "HMX - Wikipedia, the free encyclopedia". En.wikipedia.org. http://en.wikipedia.org/wiki/HMX. Retrieved 2010-05-07.
- ↑ "Nanowire battery can hold 10 times the charge of existing lithium-ion battery". News-service.stanford.edu. 2007-12-18. http://news-service.stanford.edu/news/2008/january9/nanowire-010908.html. Retrieved 2010-05-07.
- ↑ "Lithium Thionyl Chloride Batteries". Nexergy. http://www.nexergy.com/lithium-thionyl-chloride.htm. Retrieved 2010-05-07.
- ↑ "The Unitized Regenerative Fuel Cell". Llnl.gov. 1994-12-01. http://www.llnl.gov/str/Mitlit.html. Retrieved 2010-05-07.
- ↑ "Technology". SolarReserve. http://www.solar-reserve.com/technology.html. Retrieved 2010-05-07.
- ↑ "New battery could change world, one house at a time". Heraldextra.com. 2009-04-04. http://www.heraldextra.com/news/article_b0372fd8-3f3c-11de-ac77-001cc4c002e0.html. Retrieved 2010-05-07.
- ↑ "Energy Citations Database (ECD) - - Document #5960185". Osti.gov. http://www.osti.gov/energycitations/product.biblio.jsp?osti_id=5960185. Retrieved 2010-05-07.
- ↑ 18.0 18.1 18.2 "ProCell Lithium battery chemistry". Duracell. http://www.duracell.com/Procell/chemistries/lithium.asp. Retrieved 2009-04-21.
- ↑ "Properties of non-rechargeable lithium batteries". corrosion-doctors.org. http://www.corrosion-doctors.org/PrimBatt/table2.htm. Retrieved 2009-04-21.
- ↑ 20.0 20.1 20.2 20.3 20.4 "Battery energy storage in various battery types". AllAboutBatteries.com. http://www.allaboutbatteries.com/Battery-Energy.html. Retrieved 2009-04-21.
- ↑ A typically available lithium ion cell with an Energy Density of 201 wh/kg [1]
- ↑ "Lithium Batteries". http://www.globalspec.com/Specifications/Electrical_Electronic_Components/Batteries/Lithium_Batteries. Retrieved 2010-07-02.
- ↑ 23.0 23.1 Justin Lemire-Elmore (2004-04-13). "The Energy Cost of Electric and Human-Powered Bicycles". p. 7. http://www.ebikes.ca/sustainability/Ebike_Energy.pdf. Retrieved 2009-02-26. "Table 3: Input and Output Energy from Batteries"
- ↑ "Lithium Sulfur Rechargeable Battery Data Sheet". Sion Power, Inc.. 2005-09-28. http://www.sionpower.com/pdf/sion_product_spec.pdf.
- ↑ Kolosnitsyn, V.S.; E.V. Karaseva (2008). Lithium-sulfur batteries: Problems and solutions. Maik Nauka/Interperiodica/Springer. pp. 506–509. doi:10.1134/s1023193508050029.
- ↑ Storage Technology Report, ST6 Flywheel
- ↑ "Next-gen Of Flywheel Energy Storage". Product Design & Development. http://www.pddnet.com/article-next-gen-of-flywheel-energy-storage/. Retrieved 2009-05-21.
- ↑ Advanced Materials for Next Generation NiMH Batteries, Ovonic, 2008
- ↑ "ZBB Energy Corp". Archived from the original on 2007-10-15. http://web.archive.org/web/20071015134212/http://zbbenergy.com/technology.htm. "75 to 85 watt-hours per kilogram"
- ↑ High Energy Metal Hydride Battery
- ↑ "Microsoft Word - V-FUEL COMPANY AND TECHNOLOGY SHEET 2008.doc" (PDF). http://www.vfuel.com.au/infosheet.pdf. Retrieved 2010-05-07.
- ↑ "Maxwell Technologies: Ultracapacitors - BCAP3000". Maxwell.com. http://maxwell.com/ultracapacitors/products/large-cell/bcap3000.asp. Retrieved 2010-05-07.
- ↑ 33.0 33.1 http://www2.fs.cvut.cz/web/fileadmin/documents/12241-BOZEK/publikace/2004/Sup-Cap-Energy-Storage.pdf
- ↑ [2]
- ↑ http://www.doc.ic.ac.uk/~mpj01/ise2grp/energystorage_report/node9.html
- ↑ 36.0 36.1 http://www.askmar.com/Magnets/Promising%20Magnet%20Applications.pdf
- ↑ "Garage Door Springs". Garagedoor.org. http://garagedoor.org/residential/torsion-springs.php. Retrieved 2010-05-07.
- ↑ "Solutions for Hydrogen Storage and Distribution" (PDF). http://www.gov.pe.ca/photos/original/dev_solutions.pdf. Retrieved 2010-05-07.
- ↑ "Boron: A Better Energy Carrier than Hydrogen? (28 February 2009)". Eagle.ca. http://www.eagle.ca/~gcowan/boron_blast.html#TOC. Retrieved 2010-05-07.
- ↑ 40.0 40.1 40.2 40.3 Envestra Limited. Natural Gas. Retrieved 2008-10-05.
- ↑ 41.0 41.1 41.2 41.3 IOR Energy. List of common conversion factors (Engineering conversion factors). Retrieved 2008-10-05.
- ↑ 42.0 42.1 42.2 42.3 42.4 http://www.aquafoam.com/papers/selection.pdf
- ↑ http://www-static.shell.com/static/aus/downloads/aviation/avgas_100ll_pds.pdf
- ↑ "Energy Density of Aviation Fuel". Hypertextbook.com. http://hypertextbook.com/facts/2003/EvelynGofman.shtml. Retrieved 2010-05-07.
- ↑ Nature. "Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates : Abstract". Nature. http://www.nature.com/nature/journal/v447/n7147/abs/nature05923.html. Retrieved 2010-05-07.
- ↑ Justin Lemire-Elmore (2004-04-13). "The Energy Cost of Electric and Human-Powered Bicycles". p. 5. http://www.ebikes.ca/sustainability/Ebike_Energy.pdf. Retrieved 2009-02-26. "properly trained athlete will have efficiencies of 22 to 26%"
- ↑ Fisher, Juliya (2003). "Energy Density of Coal". The Physics Factbook. http://hypertextbook.com/facts/2003/JuliyaFisher.shtml. Retrieved 2006-08-25.
- ↑ http://dbresearch.com/PROD/DBR_INTERNET_EN-PROD/PROD0000000000079095.pdf
- ↑ "Energy Density of Coal". Hypertextbook.com. http://hypertextbook.com/facts/2003/JuliyaFisher.shtml. Retrieved 2010-05-07.
- ↑ "Elite_bloc.indd" (PDF). http://www.payne-worldwide.com/documents/cms/Elite_bloc_msds.pdf. Retrieved 2010-05-07.
- ↑ Bord na Mona, Peat for Energy
- ↑ http://www.ebikes.ca/sustainability/Ebike_Energy.pdf
- ↑ "energy buffers". Home.hccnet.nl. http://home.hccnet.nl/david.dirkse/math/energy.html. Retrieved 2010-05-07.
- ↑ "Biomass Energy Foundation: Fuel Densities". Woodgas.com. http://www.woodgas.com/fuel_densities.htm. Retrieved 2010-05-07.
- ↑ David E. Dirkse. energy buffers. "household waste 8..11 MJ/kg"
- ↑ http://scitation.aip.org/getabs/servlet/GetabsServlet?prog=normal&id=JESOAN000157000001000A50000001&idtype=cvips&gifs=yes
- ↑ "Technical bulletin on Zinc-air batteries". Duracell. http://www.duracell.com/oem/primary/Zinc/zinc_air_tech.asp. Retrieved 2009-04-21.
- ↑ C. Knowlen, A.T. Mattick, A.P. Bruckner and A. Hertzberg, "High Efficiency Conversion Systems for Liquid Nitrogen Automobiles", Society of Automotive Engineers Inc, 1988.
- ↑ "Energy Density - from Eric Weisstein's World of Physics". Scienceworld.wolfram.com. http://scienceworld.wolfram.com/physics/EnergyDensity.html. Retrieved 2010-05-07.
- ↑ What's the Energy Density of the Vacuum?
- ↑ "[hep-ph/0304257] Introductory review of cosmic inflation". Arxiv.org. 2003-04-28. http://arxiv.org/abs/hep-ph/0304257. Retrieved 2010-05-07.
- ↑ "[astro-ph/0005003] An Exposition on Inflationary Cosmology". Arxiv.org. http://arxiv.org/abs/astro-ph/0005003. Retrieved 2010-05-07.